| SSI Risk Assessment Tool 2022.pdf | |
| File Size: | 262 kb |
| File Type: | |
| CDC Guideline-for-Prevention-of-Surgical-Site Infections 2017 | |
| File Size: | 1036 kb |
| File Type: | |
| American College of Surgeons -Surgical Infection_Society_Update_Guidelines_2016.pdf | |
| File Size: | 202 kb |
| File Type: | |
| World Health Organization Global Guidelines to Prevent SSIs Nov 2016.pdf | |
| File Size: | 1431 kb |
| File Type: | |
| Wisconsin_Division_of_Public_Health_SSI_Prevention_Guidance__2017.pdf | |
| File Size: | 618 kb |
| File Type: | |
| APSIC - Guidelines_2019.pdf | |
| File Size: | 848 kb |
| File Type: | |
"AORN Guidelines provide guidance for establishing and maintaining a sterile field. The creation and maintenance of a sterile field can directly influence patient outcomes. Adherence to aseptic practices by all individuals involved in surgical interventions aids in fulfilling the professional responsibility to protect patients from injury. Aseptic practices are implemented pre-operative, intra-operative, and post-operative to minimize wound contamination and reduce patient risks for surgical site infections."
AORN Guidelines for Maintaining a Sterile and Sterile Technique
Visit or join AORN www.aorn.org for additional educational materials offered by the organization
Safe Operating Room
*traffic control, limit number of personnel in room
*air handling systems, calibration of system, temps and humidity, HEPA filters, maintenance
of intake and exhaust grills, air exchanges
*surgical attire (complete head coverage, double gloves, impervious gowns, arms covered)
*ensure correct processes for room turnover and terminal cleaning procedures
*ensure meticulous instrument cleaning and proper sterilization practices and biologic monitors
*storage of supplies, cleaning of supply bins, cleaning and storage of stationary equipment
*safe medication handling practices
*safe handling of laryngoscope blades and handles
*careful handling of tissues
*use of wound protectors during abdominal surgery to prevent contamination
*follow ERAS guidelines
AORN Guidelines Related to Infection Prevention in the OR
www.aorn.org
To learn more about guidelines and standards related to infection prevention, please visit the following organizations:
www.aorn.org
- Preoperative Patient Skin Antisepsis
Environmental Cleaning in the Peri-operative Setting
Surgical Tissue Banking
Surgical Hand Antisepsis and Hand Hygiene
Cleaning and Care of Instruments and Powered Equipment
High Level Disinfection
Cleaning and Processing Anesthesia Equipment
Sterilization in the Peri-operative Setting
Hand hygiene
Sterilization Technique
Antiseptic technique
Cleaning and processing of endoscopic equipment
To learn more about guidelines and standards related to infection prevention, please visit the following organizations:
- AAMI - Association for the Advancement of Medical Instrumentation
- AORN - Association of periOperative Registered Nurses
- APIC - Association for Professionals in Infection Control and Epidemiology
- IAHSCMM – International Association of Healthcare Central Service Materiel Management
- IHI - Institute for Healthcare Improvement
- JCAHO - Joint Commission on Accreditation of Healthcare Organizations
- SHEA - Society for Healthcare Epidemiology of America
- CDC - Surgical Site Infection Prevention Guidelines http://www.cdc.gov
- WHO - Guideline for Prevention of SSI
- American College of Surgeons - Guideline for Prevention of SSI
Aerobiotix Illuvia Air Purifying and Decontamination System - for Operating Room Disinfection and COVID Air Disinfection

The Illuvia system is an air-handling apparatus for a surgical operating room intended to produce a directed, nonturbulent flow of air that has been filtered to remove particulate matter and microorganisms to provide an area free of contaminants to reduce the possibility of infection in the patient.
Aerobiotix, Inc. is an American manufacturer and marketer of air disinfection systems for healthcare facilities. Our products are sold worldwide for the purpose of reducing airborne viruses and bacteria in healthcare settings. We manufacture hospital air quality systems including the Illuvia® HUAIRS a portable HEPA air filtration system designed to clean the air in hospitals. Ours systems are also known as hospital air purifiers or medical grade air purifiers. We target elimination of hospital viruses and bacteria in the air using HEPA UV-C technology.
www.aerobiotix.com
Aerobiotix, Inc. is an American manufacturer and marketer of air disinfection systems for healthcare facilities. Our products are sold worldwide for the purpose of reducing airborne viruses and bacteria in healthcare settings. We manufacture hospital air quality systems including the Illuvia® HUAIRS a portable HEPA air filtration system designed to clean the air in hospitals. Ours systems are also known as hospital air purifiers or medical grade air purifiers. We target elimination of hospital viruses and bacteria in the air using HEPA UV-C technology.
www.aerobiotix.com
| improvement_of_OR_air_quality_and_sustained_reduction_of_surgical_site_infections_in_an_orthopedic_specialty_hospital_AJIC Feb_2024.pdf | |
| File Size: | 4713 kb |
| File Type: | |
| 2021_bischoff_iche_removal_of_sarscov2_bioaerosols_using_ultraviolet_air_filtration.pdf | |
| File Size: | 677 kb |
| File Type: | |
| cook_edmiston_barnes_et_al__supplemental_air_decontamination_2019_j_arthroplasty.pdf | |
| File Size: | 357 kb |
| File Type: | |

Indigo Clean - White Light Disinfection
https://kenall.com/Indigo-Clean
Unlike other lighting disinfection technologies, Indigo‑Clean uses the visible light spectrum; it is always safe for room occupants -even when performing at the highest level of disinfection. This means rooms can remain occupied with no worries about safety.For more than 7 years, this patented lighting technology has been effectively used by hundreds of hospitals and healthcare systems to reduce harmful bacteria, such as Staph, including MRSA, in ORs and patient spaces. Indigo‑Clean has also demonstrated a reduction in intraoperative transmission and surgical site infections in studies at Maury Regional Medical Center and the University of Iowa. Most recently, it has been proven highly effective in killing viruses; expanding its use far beyond healthcare settings to include offices, schools, hospitality, retail, and other congregate settings.
Indigo‑Clean technology can help reduce the costs associated with C. diff and MRSA, as well as SARS‑CoV‑2 and Influenza‑A transmission. Considering the benefit of safely opening classrooms, stores, offices, and other group settings during the pandemic, the value is priceless.
https://kenall.com/Indigo-Clean
Unlike other lighting disinfection technologies, Indigo‑Clean uses the visible light spectrum; it is always safe for room occupants -even when performing at the highest level of disinfection. This means rooms can remain occupied with no worries about safety.For more than 7 years, this patented lighting technology has been effectively used by hundreds of hospitals and healthcare systems to reduce harmful bacteria, such as Staph, including MRSA, in ORs and patient spaces. Indigo‑Clean has also demonstrated a reduction in intraoperative transmission and surgical site infections in studies at Maury Regional Medical Center and the University of Iowa. Most recently, it has been proven highly effective in killing viruses; expanding its use far beyond healthcare settings to include offices, schools, hospitality, retail, and other congregate settings.
Indigo‑Clean technology can help reduce the costs associated with C. diff and MRSA, as well as SARS‑CoV‑2 and Influenza‑A transmission. Considering the benefit of safely opening classrooms, stores, offices, and other group settings during the pandemic, the value is priceless.
| influence_of_a_visible-light_continuous_environmental_disinfection_system_on_ortho_ssi_ajic_jan_2019_in_press.pdf | |
| File Size: | 349 kb |
| File Type: | |
Surgical Light System
http://www.americansurg.com/
The Surgical Light Drape is an innovative product and the first in the marketplace to provide sterile barrier protection for the operating room lights. The Surgical Light Drape is a low cost, single patient use, crystal clear sterile drape that adds additional protection to maintain the integrity of the sterile surgical field.
http://www.americansurg.com/
The Surgical Light Drape is an innovative product and the first in the marketplace to provide sterile barrier protection for the operating room lights. The Surgical Light Drape is a low cost, single patient use, crystal clear sterile drape that adds additional protection to maintain the integrity of the sterile surgical field.
|
First clinically-tested UVC product proven to kill pathogens on the soles of shoes.
Manage the shoe-borne spread of deadly organisms in your facility with Healthy Sole Plus®. The dirtiest, most-overlooked place in healthcare is the bottom of the shoes walking around it. Don’t let dangerous organisms travel in and out of high risk areas of healthcare ever again! Healthy Sole Plus® is the first in its industry to use UV-C technology to kill 99.9% of the germs and pathogens on the soles of shoes. Even in a clean patient room, pathogens have been proven to spread from shoes to patient skin within 30 minutes in 96% of clinical simulations. |
| study_theatre_shoes_cell_phones_in_or_tjrs.pdf | |
| File Size: | 544 kb |
| File Type: | |
CLIPVAC - Vacuum Assisted Clipper
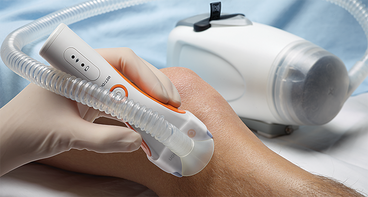
CLIPVAC - Vacuum Assisted Clipped Hair Removal For prevention on hair contamination in the OR for a safe surgical field.
http://www.carefusion.com/our-products/infection-prevention/preprocedure-hair-removal/clipvac-presurgical-hair-removal-system
The ClipVac™ pre-surgical hair removal system conveniently attaches to the Surgical Clippers to create a complete pre-surgical hair removal system, eliminating the need for extra cleanup with messy tape or mitts.
http://www.carefusion.com/our-products/infection-prevention/preprocedure-hair-removal/clipvac-presurgical-hair-removal-system
The ClipVac™ pre-surgical hair removal system conveniently attaches to the Surgical Clippers to create a complete pre-surgical hair removal system, eliminating the need for extra cleanup with messy tape or mitts.
| Spencer et al Perioperative_Hair_Removal_-_A_Review_of_Best_Practice_-_Journ_of_Periop_Practice_April_2018.pdf | |
| File Size: | 214 kb |
| File Type: | |
| Edmiston C et al. CLIPVAC Hair Removal AJIC 2016.pdf | |
| File Size: | 402 kb |
| File Type: | |
| APIC June 2016 Advances in Surgical Clipping - lecture.pdf | |
| File Size: | 1798 kb |
| File Type: | |
Wound Protector Retractors - Prevents Cross Contamination

The Medtronic (Covidien) wound protector/retractor
- Circumferential elastic retraction maximizes the working area for better exposure and visualization
- Additional exposure is achievable with the addition of the retraction ring
- Protects wounds at the incision site with 3x stronger film material than Alexis™* wound protector/retractor, with the same material thickness1
- Keeps incision moist and protects against contamination
- The proximal blue ring is not only easier to roll down compared to Alexis™* O, but can also be rolled down by just one person3
- The flexible distal grey ring is inserted through the incision in seconds and easily removed at the end of a procedur
- Large and Extra Large sizes are available with a Retraction Ring
- The SurgiSleeve™ wound protector with retraction ring provides greater retraction and visibility vs. standard wound protector
- http://www.medtronic.com/covidien/products/trocars-access/surgisleeve-wound-protector
|
| ||||||
CleanCision: WOUND PROTECTOR AND IRRIGATION SYSTEM
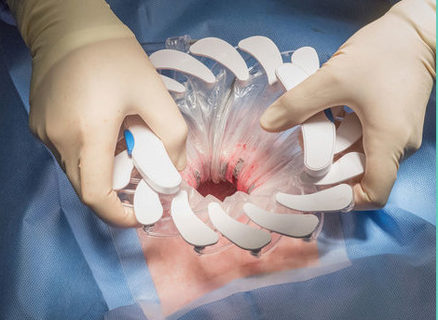
www.cleancision.com/
CleanCisionTM
A novel, first-in-class, advanced intraoperative infection control system, developed by surgeons and infection control experts, to attack and defend against the most pervasive sources of surgical site infection.
Readily incorporated into surgical and infection reduction protocols, only CleanCision has been shown to reverse pre-existing contamination1, fight and remove intraoperative contamination1 and suppress bacterial proliferation2 after incision closure.1 Papaconstantinou HT, Ricciardi R, Margolin DA, et al. A N
REFERENCES:
1 Papaconstantinou HT, Ricciardi R, Margolin DA, et al. A Novel Surgical Device Combining Continuous Intraoperative Wound Irrigation and Barrier Protection Markedly Reduces Incisional Contamination in Colorectal Surgery. Oral presentation at: American Society of Colon and Rectal Surgeons Annual Scientific Meeting; June 2017; Seattle, WA.
2 Suh I, Long SA, Coe J, Koehler J, Fry D, Welton ML. The Efficacy of a Novel Surgical Device in Preventing Intraoperative Wound Contamination in an In Vivo Porcine Model. J Laparoendosc Adv Surg Tech A. 2017
3 As shown in: Papaconstantinou HT, Ricciardi R, Margolin D, et al. Impact of Novel Wound Protection Device on Observed vs. Expected Surgical Site Infection Rates Following Colectomy Using the National Surgical Quality Improvement Program Risk Calculator. Paper presented at: WSA 2017 Annual Meeting 2017; Scottsdale, AZ.
CleanCisionTM
A novel, first-in-class, advanced intraoperative infection control system, developed by surgeons and infection control experts, to attack and defend against the most pervasive sources of surgical site infection.
Readily incorporated into surgical and infection reduction protocols, only CleanCision has been shown to reverse pre-existing contamination1, fight and remove intraoperative contamination1 and suppress bacterial proliferation2 after incision closure.1 Papaconstantinou HT, Ricciardi R, Margolin DA, et al. A N
REFERENCES:
1 Papaconstantinou HT, Ricciardi R, Margolin DA, et al. A Novel Surgical Device Combining Continuous Intraoperative Wound Irrigation and Barrier Protection Markedly Reduces Incisional Contamination in Colorectal Surgery. Oral presentation at: American Society of Colon and Rectal Surgeons Annual Scientific Meeting; June 2017; Seattle, WA.
2 Suh I, Long SA, Coe J, Koehler J, Fry D, Welton ML. The Efficacy of a Novel Surgical Device in Preventing Intraoperative Wound Contamination in an In Vivo Porcine Model. J Laparoendosc Adv Surg Tech A. 2017
3 As shown in: Papaconstantinou HT, Ricciardi R, Margolin D, et al. Impact of Novel Wound Protection Device on Observed vs. Expected Surgical Site Infection Rates Following Colectomy Using the National Surgical Quality Improvement Program Risk Calculator. Paper presented at: WSA 2017 Annual Meeting 2017; Scottsdale, AZ.
| schweizer_bmj_2013_bundles_in_ortho_surg_-_meta_analysis.pdf | |
| File Size: | 1364 kb |
| File Type: | |
| mohan._plastic_wound_retractors_as_bacteriological_barriers.pdf | |
| File Size: | 390 kb |
| File Type: | |
| lee_p._use_of_wound-protection__system_and_postoperative_wound-infect.pdf | |
| File Size: | 117 kb |
| File Type: | |
| lee_k-w._use_of_an_upper_midline__incision_for_living_donor_partial_hepatectomy.pdf | |
| File Size: | 174 kb |
| File Type: | |
| horiuchi._journal_of_trauma.__randomized_controlled_investigation_of_the_anti.pdf | |
| File Size: | 151 kb |
| File Type: | |
| edwards._wound_protectors_reduce_surgical_site_infection.pdf | |
| File Size: | 248 kb |
| File Type: | |
| colorectal_ssi_bundle__cleveland_clinic_jon_vogel.pdf | |
| File Size: | 2510 kb |
| File Type: | |
| cheng._alexis_o-ring_wound__retractor_vs_conventional_would_protection.pdf | |
| File Size: | 90 kb |
| File Type: | |
| barrier_wound_protection_decreases_ssi_in_open_elective_colorectal_surgery_cd0024.pdf | |
| File Size: | 113 kb |
| File Type: | |
| a_wound_protector_shields_incision_sites_from_bacterial_invasion_cd0022.pdf | |
| File Size: | 80 kb |
| File Type: | |
| oxygenation_and_ssi_reduction.doc | |
| File Size: | 38 kb |
| File Type: | doc |
| kansasscip101410dbratzler.pdf | |
| File Size: | 2004 kb |
| File Type: | |
| scip_-__no_correlation_to_ssi_reduction_-_ajic_july_2013.pdf | |
| File Size: | 135 kb |
| File Type: | |
| edmiston_et_al_surg_infection_2011_2.pdf | |
| File Size: | 133 kb |
| File Type: | |
| ashp_2013_surgical_prophylaxis_guidellines.pdf | |
| File Size: | 11084 kb |
| File Type: | |
| hawn_et_al_ann_surg_2011.pdf | |
| File Size: | 191 kb |
| File Type: | |
| core_measures_roles_responsibilities_v2.pdf | |
| File Size: | 971 kb |
| File Type: | |
| dirty_instruments_aug_2013.pdf | |
| File Size: | 416 kb |
| File Type: | |
| asset-surgical-instrumentation-eliminating-chaos.pdf | |
| File Size: | 542 kb |
| File Type: | |
| sample_spd_assessment_checklist_w_aami_guidelines.pdf | |
| File Size: | 89 kb |
| File Type: | |
| 2013_dec_-_ajic_-_contaminated_laparotomy_instruments.pdf | |
| File Size: | 340 kb |
| File Type: | |
| infection_control_today_-_css_guidelines.pdf | |
| File Size: | 305 kb |
| File Type: | |
| outbreak_of_pseudomonas_arthroscopic_ssi_-_iche_2011.pdf | |
| File Size: | 460 kb |
| File Type: | |
| survival_of_bacteria_on_scrubs.pdf | |
| File Size: | 49 kb |
| File Type: | |
| shea_ssi_-_2009.pdf | |
| File Size: | 252 kb |
| File Type: | |
| ihi_howtoguidepreventssi.pdf | |
| File Size: | 581 kb |
| File Type: | |
| ihi_project_joints_-_chg_bathing.ppt | |
| File Size: | 2344 kb |
| File Type: | ppt |
| ihi_project_joints-sa_screening.ppt | |
| File Size: | 2401 kb |
| File Type: | ppt |
| ihi_project_joints-_alcohol_skin_prep.ppt | |
| File Size: | 2346 kb |
| File Type: | ppt |
| fletcher._prevention_of_perioperative_infection.pdf | |
| File Size: | 646 kb |
| File Type: | |
| hair_as_a_reservoir_for_staphlococci.pdf | |
| File Size: | 522 kb |
| File Type: | |
| anesth_analgesia_2011_hand_contamination___.pdf | |
| File Size: | 1416 kb |
| File Type: | |
| charles_edmiston_-_pevzner_et_al_obst_gynecol_2011.pdf | |
| File Size: | 261 kb |
| File Type: | |
| reducing_ssi_with_alexis_wound_protectorretractor_feb_2013.pdf | |
| File Size: | 1187 kb |
| File Type: | |
| or_air_contamination_-_edmiston_-_surgery_2005.pdf | |
| File Size: | 408 kb |
| File Type: | |
| surgical_gloves_-_edmiston_et_al_ajic_2011.pdf | |
| File Size: | 207 kb |
| File Type: | |
| proposed_2020_acute_care_hospitals_hai_action_plan_targets.pdf | |
| File Size: | 1048 kb |
| File Type: | |

