| AORN_2016_-Spencer et al_Wound_Prevalence_Study_Poster_pdf3.pdf | |
| File Size: | 233 kb |
| File Type: | |
| Topical Skin Adhesive Microbial-Barrier-White-Paper-final.pdf | |
| File Size: | 85 kb |
| File Type: | |
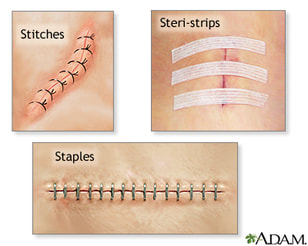
There are many types of surgical incision closure - stitches, steri-strips, staples and topical incisional adhesives. Some surgeons protect the incision with antimicrobial dressings.
Some studies suggest an increased risk of dehiscence and infection with the use of staples and stitches due to the potential for exogenous contamination from the environment (hospital or home setting).
Topical skin adhesive (TSA) (octyl cyanoacrylate) provide a strong flexible bond that lasts approximately 7-10 days. It eliminates staple removal, can reduce potential for suturing injuries to staff, provides a flexible bonding strength and microbial barrier to the incision.
Not all incisions can be closed with TSA - and an added protection to a newly healing incision can be the use of antimicrobial dressings - such as PHMB gauze dressing and silver dressings
Some studies suggest an increased risk of dehiscence and infection with the use of staples and stitches due to the potential for exogenous contamination from the environment (hospital or home setting).
Topical skin adhesive (TSA) (octyl cyanoacrylate) provide a strong flexible bond that lasts approximately 7-10 days. It eliminates staple removal, can reduce potential for suturing injuries to staff, provides a flexible bonding strength and microbial barrier to the incision.
Not all incisions can be closed with TSA - and an added protection to a newly healing incision can be the use of antimicrobial dressings - such as PHMB gauze dressing and silver dressings
| bmj_2010_sutures_versus_staples_for_skin_closure.pdf | |
| File Size: | 146 kb |
| File Type: | |
| basha_2010_am_j_obstet_gynecol.pdf | |
| File Size: | 196 kb |
| File Type: | |
| tuuli_obstet_gynecol_2011_-_complications_with_staples.pdf | |
| File Size: | 4299 kb |
| File Type: | |
Dermabond Advanced
www.jnjmedicaldevices.com/en-US/product/dermabond-advanced-topical-skin-adhesive
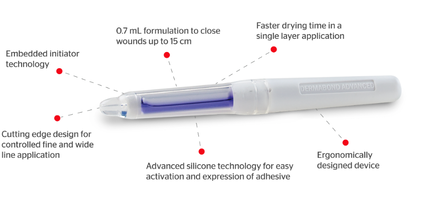
DERMABOND ADVANCED™ Topical Skin Adhesive is a sterile, liquid topical skin adhesive containing a monomeric (2-octyl cyanoacrylate) formulation and the colorant D & C Violet #2. It is provided as a single-use applicator in a blister package. The pen style applicator is composed of a crushable ampoule contained within a plastic applicator. As it is applied to the skin, the liquid is syrup-like in viscosity and polymerizes within minutes. Studies have shown that following application, DERMABOND ADVANCED™ Adhesive acts as a barrier to prevent microbial penetration as long as the adhesive remains.
INDICATIONS
DERMABOND ADVANCED™ Adhesive is intended for topical application only, to hold closed easily approximated skin edges of wounds from surgical incisions, including incisions from minimally invasive surgery, and simple, thoroughly cleansed, trauma-induced lacerations. DERMABOND ADVANCED™ Adhesive may be used in conjunction with, but not in place of, deep dermal stitches.
CONTRAINDICATIONS
Do not use on any wound with evidence of active infection, gangrene, or wounds of decubitus etiology.
Do not use on mucosal surfaces or across mucocutaneous junctions (e.g., oral cavity, lips), or on skin which may be regularly exposed to body fluids or with dense natural hair.
Do not use on patients with a known hypersensitivity to cyanoacrylate or formaldehyde. http://dermabond.com
INDICATIONS
DERMABOND ADVANCED™ Adhesive is intended for topical application only, to hold closed easily approximated skin edges of wounds from surgical incisions, including incisions from minimally invasive surgery, and simple, thoroughly cleansed, trauma-induced lacerations. DERMABOND ADVANCED™ Adhesive may be used in conjunction with, but not in place of, deep dermal stitches.
CONTRAINDICATIONS
Do not use on any wound with evidence of active infection, gangrene, or wounds of decubitus etiology.
Do not use on mucosal surfaces or across mucocutaneous junctions (e.g., oral cavity, lips), or on skin which may be regularly exposed to body fluids or with dense natural hair.
Do not use on patients with a known hypersensitivity to cyanoacrylate or formaldehyde. http://dermabond.com
| octyl_cyanoacrylate_for_cervical_and_lumbar_surgery_2010_neurosurg_rev.pdf | |
| File Size: | 158 kb |
| File Type: | |
| dermabond_slides.ppt | |
| File Size: | 1838 kb |
| File Type: | ppt |
| dermabond_-_instructions_for_use.pdf | |
| File Size: | 142 kb |
| File Type: | |
| dermabond_and_prineo.ppt | |
| File Size: | 7064 kb |
| File Type: | ppt |
| prineo_application.ppt | |
| File Size: | 1291 kb |
| File Type: | ppt |
AquaCel Ag (Silver) - Convatec
www.convatec.com/search/?q=AquaCel+Silver
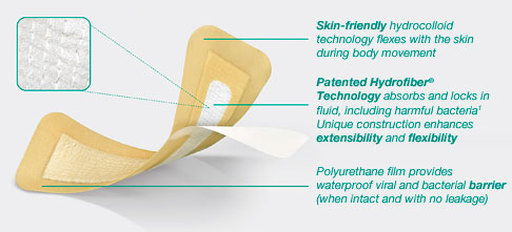
Combination dressing with a mix of skin-friendly hydrocolloid technology for comfort during body movement, and proprietary Hydrofiber® Technology with ionic silver to help manage serosanguinous fluid. Cover layer of polyurethane film provides a viral, waterproof and bacterial barrier (when intact and with no leakage), for a high-performance dressing. Added ionic silver provides sustained antimicrobial activity for up to fourteen days as demonstrated by in vitro studies.
| a_prospective_two-armed_trial_assessing_silver_dressing_performance_postoperatively_schwartz_et_al_owm_2014.pdf | |
| File Size: | 1173 kb |
| File Type: | |
| naon_-_use_o_silver_dressing_for_spine_incisions_poster0506.pdf | |
| File Size: | 165 kb |
| File Type: | |
| aquacel_surgical_dressing_reduces_the_rate_of_acute_pji_following_total_joint_cai_et_al_joa_2014.pdf | |
| File Size: | 176 kb |
| File Type: | |
| 3m_postop_dressing.pdf | |
| File Size: | 1118 kb |
| File Type: | |
Antimicrobial Gauze Dressings (AMD) - Cardinal Health
https://www.cardinalhealth.com/en/product-solutions/medical/skin-and-wound-management/antimicrobial-dressings.html?cid=SOC-YTB-MED-MP-NCP-SWM-AMDwPHMB-012920

Kendall™ AMD antimicrobial dressings are part of a proven prophylactic infection prevention program. Unlike ordinary sterile dressings which do not provide an effective barrier to bacterial invasion, Kendall™ AMD Antimicrobial Dressings contain 0.2% Polyhexamethylene Biguanide (PHMB), a bacteria-killing polymer that virtually eliminates bacterial penetration through and growth within the dressings.
The Kendall™ AMD antimicrobial family of dressings are effective against both gram + and gram - bacteria as well as yeast and fungi. They are also effective against common problematic bacteria such as methicillin resistant Staphlococcus aureus (MRSA) and
vancomycin resistant Enterococcus (VRE).
The Kendall™ AMD antimicrobial family of dressings are effective against both gram + and gram - bacteria as well as yeast and fungi. They are also effective against common problematic bacteria such as methicillin resistant Staphlococcus aureus (MRSA) and
vancomycin resistant Enterococcus (VRE).
|
| ||||||||||||
| Antimicrobial_Gauze_Dressings_naon_05-2010.pdf | |
| File Size: | 1688 kb |
| File Type: | |

